Abstract
Introduction Tafasitamab, is a humanized, Fc-modified anti-CD19 monoclonal antibody that functions as an immunotherapy through direct cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP). Based on data from the ongoing, open-label, multicenter, single-arm, Phase II L-MIND study (NCT02399085; Salles, et al. Lancet Oncol 2020), tafasitamab in combination with lenalidomide (LEN) was granted accelerated approval in the US (2020) and conditional/accelerated approval by the EMA (2021) and other regulatory authorities for the treatment of adult patients with R/R DLBCL not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are ineligible for autologous stem cell transplant (ASCT). It is a preferred regimen in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for this setting.
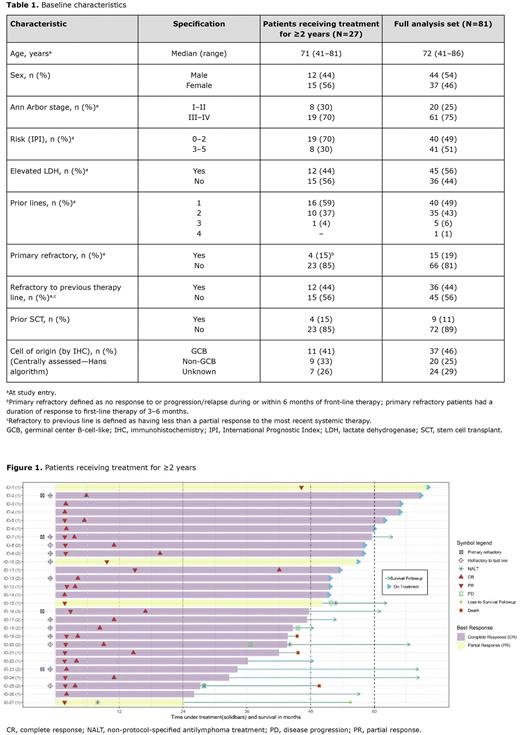
We have previously reported long-term, durable clinical activity for tafasitamab + LEN (Duell, et al. Haematologica 2021). Here, we report efficacy and safety data for tafasitamab + LEN in patients with R/R DLBCL enrolled on L-MIND who received treatment for ≥2 years and those in follow-up for ≥5 years.
Methods Patients were aged ≥18 years with ASCT-ineligible R/R DLBCL, 1-3 prior systemic therapies (including ≥1 CD20-targeting regimen), and an Eastern Cooperative Oncology Group performance status of 0-2. Patients received 28-day cycles of tafasitamab (12 mg/kg IV), once weekly during Cycles 1-3, with a loading dose on Day 4 of Cycle 1, then every 2 weeks (Q2W) during Cycles 4-12. LEN (25 mg orally) was administered on Days 1-21 of Cycles 1-12. After Cycle 12, progression-free patients received tafasitamab Q2W until disease progression. Time-to-event, treatment response (Cheson 2007), and safety endpoints were assessed.
Results At data cut-off (February 15, 2022), of the 81 patients in the full analysis set, 30 patients completed 12 cycles of tafasitamab + LEN and 4 patients discontinued LEN before 12 cycles; 27 (34%) received treatment for ≥2 years (median: 4.3 years). The proportion of patients who were primary refractory, refractory to previous therapy, or who had received prior ASCT were similar between patients who received treatment for ≥2 years and the full analysis set (Table 1). Of the 27 patients who received treatment for ≥2 years, 23 are confirmed alive, one was lost to follow-up, one died by unknown cause, and two died following adverse events (AEs) unrelated to study treatment. Thirteen patients remained on treatment; reasons for tafasitamab discontinuation after ≥2 years were progressive disease (n=4), patient/physician decision (n=8), and non-treatment-related fatal AEs (n=2: one each for COVID-19 and cardiovascular AE). Twelve patients have been in follow-up for ≥5 years, of whom six are still on treatment, while 6 had discontinued treatment although still in response.
In patients who received treatment for ≥2 years, 23 had previously achieved a complete response (CR) as a best response and four had a partial response (PR), as assessed by the investigator (Figure 1); the 48-month overall survival (OS) rate was 92.6%; however, the median duration of response, progression-free survival, and OS have not been reached. In patients refractory to previous therapy line (n=12), 91.7% were in follow-up at 48 months. All of the primary refractory patients (n=4) are in follow-up at 60 months. Of the six patients who received treatment for ≥5 years, five achieved CR (one of whom had triple-hit R/R DLBCL) and one had a PR (investigator-assessed).
A lower incidence of AEs was reported during the tafasitamab monotherapy phase (Cycles 13-24 and Cycles ≥25) compared with the tafasitamab + LEN combination therapy phase (Cycles 1-12). The exposure-adjusted incidence of AEs showed that the majority were Grade 1-2.
Conclusion Combination therapy with tafasitamab plus lenalidomide followed by tafasitamab monotherapy provided durable responses in patients with R/R DLBCL ineligible for ASCT. These long-term data suggest that this immunotherapy can achieve prolonged remission and survival of 5 years or longer in this patient population. The incidence of AEs decreased as patients transitioned from combination therapy to tafasitamab monotherapy, with long-term tafasitamab (up to 60 months) being well tolerated with no new safety signals.
Funding MorphoSys AG
Disclosures
Duell:MorphoSys: Consultancy, Research Funding; Incyte: Consultancy; Regeneron: Research Funding. Jurczak:Bayer: Research Funding; Celgene: Research Funding; TG Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Takeda: Research Funding; Lilly: Consultancy, Research Funding; Mei Pharma: Research Funding; AstraZeneca: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Sandoz: Consultancy, Research Funding; Merck: Research Funding; Morphosys: Research Funding; Novo Nordisk: Research Funding. Liberati:iQVIA: Research Funding; PSI: Research Funding; Verastem: Research Funding; Takeda: Research Funding; Secura Bio: Research Funding; Sanofi: Research Funding; Roche: Research Funding; Novartis: Research Funding; Morphosys: Research Funding; Janssen: Research Funding; DR REDDY'S LABORATORIES SPA: Research Funding; Celegene: Research Funding; BMS: Research Funding; Beigene: Research Funding; AbbVie: Research Funding; LOXO: Research Funding; MEI-PHARMA: Research Funding; EPZIME: Research Funding. Halka:Not applicable: Other: Not applicable. Carbó:Not applicable: Other: Not applicable. Abrisqueta Costa:Roche: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Consultancy, Honoraria, Speakers Bureau; Sandoz: Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau. Maddocks:Pharmacyclics: Consultancy, Research Funding; Lilly: Consultancy; Kite: Consultancy; Incyte: Consultancy; Gilead: Consultancy; ADC Therapeutics: Consultancy; Abbvie: Consultancy; Pfizer: Research Funding; Acerta: Consultancy; BMS: Consultancy, Research Funding; Janssen: Consultancy; Genentech: Consultancy; Morphosys: Consultancy; Genmab: Consultancy; Celgene: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy. Dreyling:Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche: Consultancy; Amgen, Astra Zeneca, Gilead/Kite, Janssen, Lilly, Novartis, Roche: Honoraria; Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche: Research Funding. Rosenwald:Not applicable: Other: not applicable. Bakuli:MorphoSys AG: Current Employment, Other; Division of Infectious Diseases and Tropical Medicine, University Hospital, Ludwig-Maximilians-Universität Munch: Consultancy. Amin:MorphoSys AG: Current Employment; Paion AG: Current equity holder in private company. Gurbanov:Not applicable: Other. Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal